
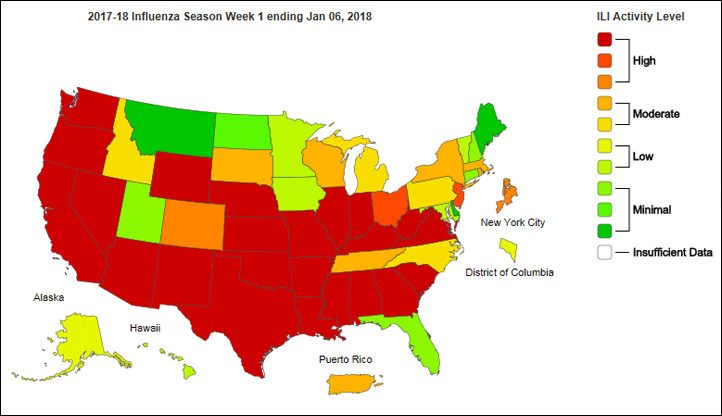
The most recent Weekly Influenza Surveillance Report by the Influenza Division at the Centers for Disease Control and Prevention shows high levels of outpatient visits to healthcare providers for influenza-like illness in most of the country.
With influenza reported as widespread in 49 of the 50 states, the Centers for Disease Control and Prevention (CDC) on Tuesday announced that the national supply of antivirals can meet the “high demands from this season.”
However, “spot shortages” have been reported in areas with “high influenza activity,” the agency’s Alicia Fry stated during Tuesday’s Public Health Grand Rounds.
Fry, with CDC’s Influenza Division, said the agency is “working closely with the manufacturers to address any existing gaps” in supply.
She referred the public to CDC’s Antiviral Drug Supply webpage for information on contacting manufacturers to inquire about the availability of the three FDA-approved neuraminidase inhibitors — oseltamivir, zanamivir, and peramivir — that are active against the influenza viruses circulating this season.
Only oseltamivir is manufactured in a formulation for oral administration.
Fry said CDC advises clinicians who prescribe influenza antivirals to consider telling patients to contact their pharmacy about its supply before making the trip.








